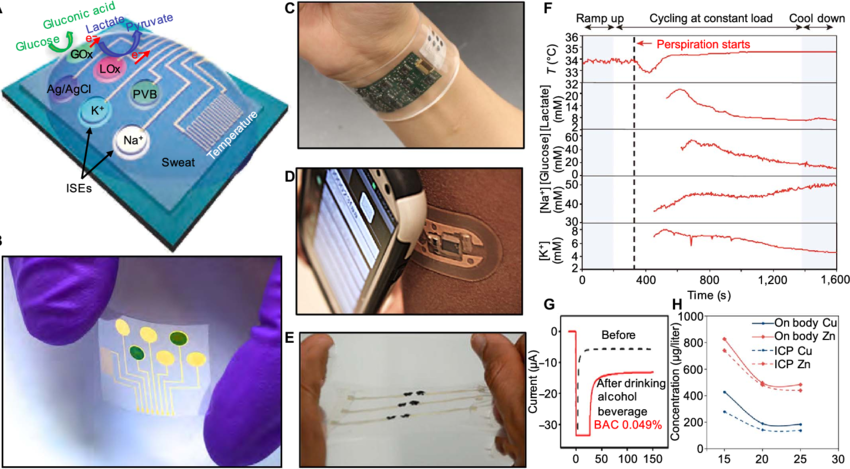
Flexible Epidermal Patch Monitors Chemicals in Sweat
Researchers at UC San Diego have developed a flexible all-in-one epidermal patch that could monitors a variety of chemicals in sweat. The new wearable could help doctors paint a picture of a person’s health and wellbeing.
The system combines skin-adherable sampling, fluidics, and detection in a unified device. It also addresses issues like contamination of the skin surface, sweat carry-over and signal regeneration.
Glucose
Researchers at Penn State have developed a sensor that can monitor glucose by drawing the sugar from the interstitial fluid between cells, as if they were using a blood test strip. The device could eliminate the need for millions of diabetics to take frequent finger-prick blood tests, according to an article published in the journal Biosensors and Bioelectronics.
The sensor is made of a microfluidic collection layer and a detector. The microfluidic collection layer may be serpentine in shape or comprise concentric microfluidic channels. The detector may be an electrochemical glucose-sensitive detector or some other suitable type of glucose detection element. The collection layer is encased in an adhesive substrate. The substrate may be a woven or non-woven flexible sheet, or it may be an adhesive polymer. The substrate may be coated with a release liner to protect the adhesive surface during storage and prior to use.
When the glucose-sensitive monomer is exposed to a physiologically significant concentration of glucose, it will fluoresce with a wavelength at which the glucose detector responds. In some variations, the glucose-sensitive monomer may be dispersed throughout the collection layer, or it may be immobilized within a reagent layer. The reagent layer may also be a polymer or other substance that is sensitive to glucose, and the reagent layer may be in fluid communication with the collection layer.
Other sensors that can measure glucose are currently available, but these devices require the patient to take a sample of sweat or other bodily fluid to be measured. This method can be uncomfortable and time-consuming. In addition, the sample can be difficult to store and analyze.
In 2022, Know Labs will launch a wristband that is positioned as the first noninvasive continuous glucose monitoring (CGM) system in the world. Called the UBand, it will send data directly to a smartphone app.
However, the UBand is unlikely to replace current glucose meters. Diabetes industry analyst Barry Ginsberg, who runs Diabetes Technology Consultants in New Jersey, says the COVID-19 pandemic has accelerated the trend toward less-invasive glucose testing. He predicts that wearable glucose monitors will soon become the norm, but cautions that it isn’t easy to develop a product that can deliver accurate results and be used by people with limited mobility or who have difficulty with finger-pricking.
Lactate
A microneedle patch to Monitors interstitial fluid (ISF) lactate has been developed and validated in human volunteers. The device is worn on the skin surface and readings closely follow venous lactate measurements. It could support clinical decision-making and bypass the need for blood testing in a range of hospital and community healthcare settings.
The researchers designed a hybrid device consisting of two silver iontophoretic electrodes and an enzymatic lactate biosensor on a double-sided medical tape that adheres to the skin surface. A disc of agarose hydrogel serves as a physical barrier between the iontophoretic electrodes and ISF, preventing ion transfer to the body’s tissues.
To test the performance of the Monitors, volunteers performed a 30-min cycling activity with the ISF monitoring patch attached to one arm. The BP and sweat-lactate data were recorded and then validated using commercial cuff-style BP and lactate monitors. The BP and sweat-lactate readings during the exercise closely followed the venous values, indicating good ISF sampling.
During rest, there was a slight lag between the ISF lactate reading and venous lactate measurement. This suggests that ISF lactate clearance is slower in the skin compared to the blood. To investigate this further, the team developed a microneedle patch to continuously measure ISF lactate. The patch consists of a polycarbonate base with protrusions that extend less than 1 mm into the skin layer. These are connected by wires to a potentiostat and a real-time readout of electrical current. A layer of the enzymatic biosensor (lactate oxidase) binds to ISF lactate, resulting in a current reduction that varies proportionally with ISF lactate concentration.
The microneedle patch was also tested with multiplexed readings, including glucose and urea. The results were similar, showing that multiplexed sweat analysis is feasible on the ISF-monitoring platform. The researchers are now working to improve the device’s performance by extending its operation time, enhancing the sensor’s ability to detect urea, and increasing the number of analytes that can be detected simultaneously.
The researchers plan to use the device for continuous and wireless monitoring of a variety of health parameters in people with chronic conditions. They hope to expand the research to include indicators of disease onset, enabling early intervention that may prevent a health event from progressing into a disease.
Caffeine
A wearable patch that monitors glucose, lactate, caffeine and alcohol levels has been developed by engineers at the University of California San Diego. The stretchable two-by-two-inch patch, which has electronic circuits printed on it that look like the face of a cartoon character with large eyes and a mustache, can be worn by people of all ages. It uses ultrasound transducers to measure blood pressure and heart rate, while chemical sensors on the skin surface detect biomarkers including those mentioned.
A cross-section of the patch is shown in FIG. 2. The device includes an occlusive layer that inhibits evaporative loss from the skin and promotes sweat accumulation into the patch collection layer. A microfluidic channel within the collection layer is in fluid communication with a buffer reservoir and is connected to the skin by a sweat permeable membrane. A valve (224) is located between the buffer reservoir and the collection layer, and is in communication with a sensor for detecting a component of the sweat. A GOx sensor, for example, is located in the collection layer. The operation of the sensor and the addition of a compound to induce sweating may be controlled by a controller, which can be part of the patch or part of a separate measurement device.
The patch is powered by a battery and communicates with the outside world via an RF antenna. It can be either disposable or reusable. The electronics are affixed to the patch’s flexible substrate, which is typically made from polyethylene terephthalate. The patch can also include a variety of other sensors, including temperature and humidity. The sensors are paired with sophisticated software that can paint a picture of the user’s condition and vital signs. The system can also transmit the data to a remote computer for analysis. The technology could be used to diagnose and monitors conditions such as diabetes, high cholesterol, and chronic cardiovascular disease, or to help patients lose weight and stop drinking or smoking. It also could be used in athletic training and to help patients with Parkinson’s disease improve their balance and coordination.
Alcohol
Researchers have long sought to make it easier to Monitors the body’s most vital signs. Current methods involve putting catheters into the bloodstream or tethering patients to multiple hospital monitors. Engineers at UC San Diego, however, have designed a flexible patch that can detect chemicals and other signals deep inside the body. The stretchable two-by-two-inch patch, which looks like a cartoon character with large eyes and a mustache, contains two chemical sensors and a blood pressure sensor.
The sensor on the left side of the patch senses lactate, caffeine and alcohol in sweat, which can be a good indicator of the concentration of these substances in the bloodstream. The right-side electrode uses a drug called pilocarpine to induce sweat. The skin absorbs the drug, and a mild electrical current passes through the sweat to produce an electric signal that is read by the sensor.
To test the system, volunteers wore the patch while performing a series of tasks. The measurements taken by the patch closely matched those collected by commercial monitoring devices, including a BP cuff, a blood lactate meter and a breathalyzer. The resulting data also showed that the device accurately tracked changes in blood pressure and heart rate during exercise and after eating a high-sugar meal.
In another experiment, the researchers tested the sensor with alcohol. The volunteer drank a single alcoholic beverage and then wore the patch while recording BP, ISF-glucose and sweat alcohol. The results from this testing show that the patch can detect a significant increase in BP during the 20 minutes following alcohol consumption, as well as a parallel rise in glucose and ISF-glucose levels. The sweat alcohol level can also be correlated with the results of a commercial breathalyzer to indicate the wearer’s blood alcohol concentration.
The researchers plan to continue developing the device. They hope to add a perspiration-rate sensing interface to address the possibility of ISF dilution and to use longer-lasting sweat-stimulating drugs (such as carbachol) in place of pilocarpine. They hope that the combination of these features could lead to a compact, flexible, wearable device for continuous noninvasive monitoring of BP, heart rate and blood sugar, as well as nutrient and metabolite levels in the sweat.