Recent Microbiome Discoveries Change Our View of Life on Earth
Researchers have found microbes in acidic pools at abandoned mines, the contaminated soil around 31,000-year-old baby teeth and inside the guts of premature infants. The latest discoveries are changing our view of what it means to be alive on Earth.
Scientists have spotted 35 new groups, or phyla, of bacteria, including some with unusual genes and the ability to survive without oxygen. These new taxa help scientists clarify a hazy branch of the tree of life.
The Microbiome
Microbiome research has come to a tipping point, with investigators rapidly shifting from simply characterizing communities to understanding how they function. These insights will have profound ramifications for human health and environmental protection.
Our microbiomes are a result of our genetics and the environment we live in. The initial composition of the microbiome is determined by the microorganisms in a newborn’s birth canal and the bacteria found in the mother’s milk. Subsequently, the microbiome evolves through interactions with other organisms and diet. The microbial community is highly personalized and the uniqueness of an individual’s microbiota is one of the reasons it has been difficult to correlate microbial diversity with specific diseases.
But the ability to conduct long-term characterization of the microbiome may help resolve this puzzle. By determining the timing of changes in microbial diversity, scientists can study whether these fluctuations are caused by the onset or remission of clinical symptoms. This information would also allow researchers to understand the mechanisms that cause certain diseases by examining how the microbiome responds to environmental factors and microbial exposures.
Using techniques such as next-generation sequencing, scientists can now examine the makeup of entire microbiome populations. In doing so, they are able to detect the presence of individual bacterial species as well as their abundance. This approach reveals important evolutionary dynamics and provides clues as to how microbial communities adapt to their environment.
As the research on microbial genomics has progressed, researchers have been able to create models that describe the complex relationships between microbes and their host ecosystems. These models are helping to inform the design of microbial experiments and the creation of new genomic tools that enable scientists to better analyze and interpret microbial data.
The use of these new genomic tools has made it possible for investigators to uncover unexpected relationships between microbes and their hosts. For example, sequence analysis of the bacteria in the digestive tract of the Tyrolean Iceman (Otzi), a 3,300-year-old body frozen in rock, revealed that some of the bacteria present in Otzi’s colon and stomach were also in the colon of modern humans.
Microbes in Space
As astronauts plan longer-duration missions to Mars, it is crucial to understand how their microbiome—a collection of microbial communities that reside in various anatomical sites in the body and play a vital role in human health and behavior—adapts to the unique stresses of spaceflight. Two primary stress conditions—radiation and microgravity —are known to disrupt the microbes in a crew member’s body, and elucidating how the organisms adapt to these conditions can help us develop countermeasures against them.
Scientists have been studying the effects of spaceflight on microbes for more than 50 years, but most of that research was performed in simulated low-gravity labs and did not take into account the specifics of spaceflight itself. To fill this knowledge gap, PNNL scientists worked with microbiologists at the International Space Station (ISS) to study how bacteria in ISS facilities respond to radiation and to varying levels of microgravity.
The team isolated and sequenced the genomes of bacteria from different locations inside the ISS. They found that most of the microbes were human-associated, including Staphylococcus, which is commonly found on the skin and in the nasal passage; Pantoea, which lives in the gastrointestinal tract; and Bacillus, a common soil-dwelling bacterium. The researchers also compared the strains of Staphylococcus and Bacillus to those from Earth and the ISS and found that they had adapted to radiation by changing their protein structures.
They also observed changes in the structure of bacteria, finding that some grew into biofilms, which encapsulate individual cells and make them more resistant to disinfectants, antibiotics and other environmental stresses. These observations are important because biofilms can cause serious infections in humans. In addition, they are difficult to detect by traditional methods of microbial monitoring.
One of the most exciting findings from their work was that a certain strain of Deinococcus bacteria—listed in Guinness World Records as the toughest bacterium—grew and survived in space for three years. This is a major step towards understanding the mystery of how life on Earth blossomed, as it supports the theory that life can travel between planets inside meteorites or comets.
Microbes on Earth
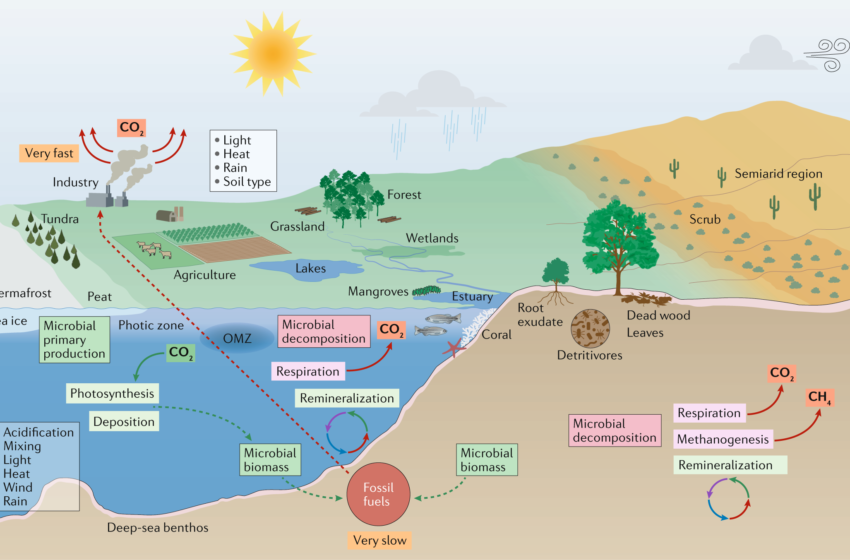
The microbial world is incredibly diverse. Bacteria, archaea and protists fill all habitats that can be occupied by larger organisms, from the frozen depths of the Arctic Ocean to the hot water shooting forth from hydrothermal vents. They are the sole life forms in extreme environments, and they play essential roles in carbon and nutrient cycling, animal (including human) and plant health, and agriculture.
Microorganisms are also indispensable for understanding our planet’s history. The oldest fossil records – including a few that suggest life on Mars – are microbial in origin. These single-celled organisms drive nutrient cycles, break down organic matter and produce oxygen, among other functions. They may cause infections, but most are neutral or even helpful. Scientists hope to learn more about these invisible yet indispensable communities in order to unlock the secrets of our own origins, and perhaps help guide our search for other Earths.
Microbial communities are highly sensitive to environmental changes. Nutrient inputs from air, river and estuarine flows affect community composition and function, and climate change alters them as well. For example, higher CO2 levels in the atmosphere can lead to decreased microbial diversity, which reduces the ability of microorganisms to transform organic carbon and nitrogen to soluble forms that are used by plants. Rising temperatures can also alleviate iron limitation of the cyanobacteria that fix nitrogen in the ocean, and this has potentially profound implications for the new nitrogen supplied to future warming ocean food webs123.
Moreover, because of their very short generation times – months to over 100 years for some bacteria – microorganisms can survive long after dead, buried macroorganisms. For example, a 2018 study published in Geobiology found that low densities of bacteria – although not exactly “dead” — persisted in sediments from 5 to 30 million years old two kilometers below the surface of the Pacific Ocean, with the microorganisms still actively, if slowly, living.
Human activities cause unprecedented animal and plant extinctions and loss of biodiversity1. Microorganisms are also susceptible to these environmental changes, but their effects are less visible and well-understood. Therefore, we need to redouble our efforts to understand and protect microorganisms. To do this, we need to better characterize microbial responses to climate change and make sure that this knowledge is integrated into frameworks for addressing global climate change and accomplishing the UN Sustainable Development Goals2.
Microbes in Your Gut
Trillions of microorganisms (mainly bacteria) live inside you. They make up what is called your gut microbiome. The microorganisms that live in your intestines are the most studied.
They are involved in many important functions, including digesting food, absorbing nutrients and producing vitamins and enzymes that you cannot produce on your own. Microbes also provide important support to your immune system, regulate body weight and help with brain functions and mood.
The bacterial community that lives in your intestines, especially the large intestine (where foods are fermented after digestion), is very different from the microbial communities that live in your stomach or small intestine. That’s because the anaerobic bacteria that live in your colon require low oxygen conditions to thrive. The higher oxygen, faster movement and strong digestive juices of the upper GI tract prevent them from colonizing there. But they have some important functions to perform in the colon, such as breaking down indigestible plant fibers and producing essential fatty acids and other nutrients that you can’t get from food.
It turns out that a person’s diet can have a huge impact on the diversity of their gut microbiome. People on hunter-gatherer diets, for example, have a much more diverse gut microbiome than those on less varied Western diets that are heavy in processed foods. A person’s microbes can also be affected by chemical exposures such as alcohol, cigarette smoke and certain medications like antibiotics.
It’s not surprising that we are only now beginning to understand how important the microbes in our intestines really are. The old saying “you are what you eat” is true when it comes to gut microbes. A healthy, diverse diet supports the health of your microbiome, and a well-developed microbiome is linked to good health. But vilifying all bacteria because of a few that cause disease is not helpful. It would be as absurd as putting every little boy selling lemonade at a corner
or all the grandmothers baking cakes on the FBI’s most wanted list. Instead, it’s better to recognize the difference between the good and the bad.